Clonazepam Recall 2024 Tacoma
Clonazepam Recall 2024 Tacoma. Specifically, endo's ongoing investigation has identified the possibility that the clonazepam product lots listed below contain a limited number of cartons printed with the. 19, 2024, by endo, inc.
The expanded recall involves clonazepam orally disintegrating tablets, with strength in usp 0.125 mg, 0.25 mg, 1 mg, and 2mg, packaged in cartons containing 60 tablets. Specifically, endo’s ongoing investigation has identified the possibility that the clonazepam product lots listed below contain a limited number of cartons printed with the.
Clonazepam Recall 2024 Tacoma Images References :
 Source: www.rttnews.com
Source: www.rttnews.com
Endo USA Recalls Clonazepam Orally Disintegrating Tablets, On july 16th, 2024, endo usa, inc.
 Source: www.drugs.com
Source: www.drugs.com
FDA Alert Endo USA, Inc. Issues Recall of One Lot of Clonazepam Orally Disintegrating Tablets, A voluntary recall notice for clonazepam orally disintegrating tablets was expanded on nov.
 Source: www.pharmanow.live
Source: www.pharmanow.live
Endo USA Recalls Clonazepam Pharma Now Pharma Now, Food and drug administration (fda) announced a recall of some lots of orally disintegrating clonazepam tablets (the generic form of klonopin), due to package.
 Source: es.oldmedic.com
Source: es.oldmedic.com
Efectos secundarios, interacciones, usos e impronta del fármaco de klonopina, oblea de klonopina, Endo inc., based in pennsylvania, has announced a voluntary recall of 16 lots of clonazepam orally disintegrating tablets after it discovered that a number of cartons may.
 Source: es.oldmedic.com
Source: es.oldmedic.com
Efectos secundarios, interacciones, usos e impronta del fármaco de klonopina, oblea de klonopina, To date, endo has not received any reports of adverse events associated with this product lot recall.
 Source: www.medwastemngmt.com
Source: www.medwastemngmt.com
FDA Recall of Clonazepam MedWaste Management, Specifically, endo's ongoing investigation has identified the possibility that the clonazepam product lots listed below contain a limited number of cartons printed with the.
 Source: www.recallguide.org
Source: www.recallguide.org
Clonazepam Information, Side Effects, Warnings and Recalls, Ndoi) (“endo”) announced today that one of its operating subsidiaries, endo usa, inc., is expanding its previously announced.
 Source: www.medwastemngmt.com
Source: www.medwastemngmt.com
MedWaste Management. Medical Waste Disposal in California, Consumers with any unused prescribed 60 tablet cartons of recalled clonazepam orally disintegrating tablets, usp 0.25mg which may also appear as clonazepam orally.
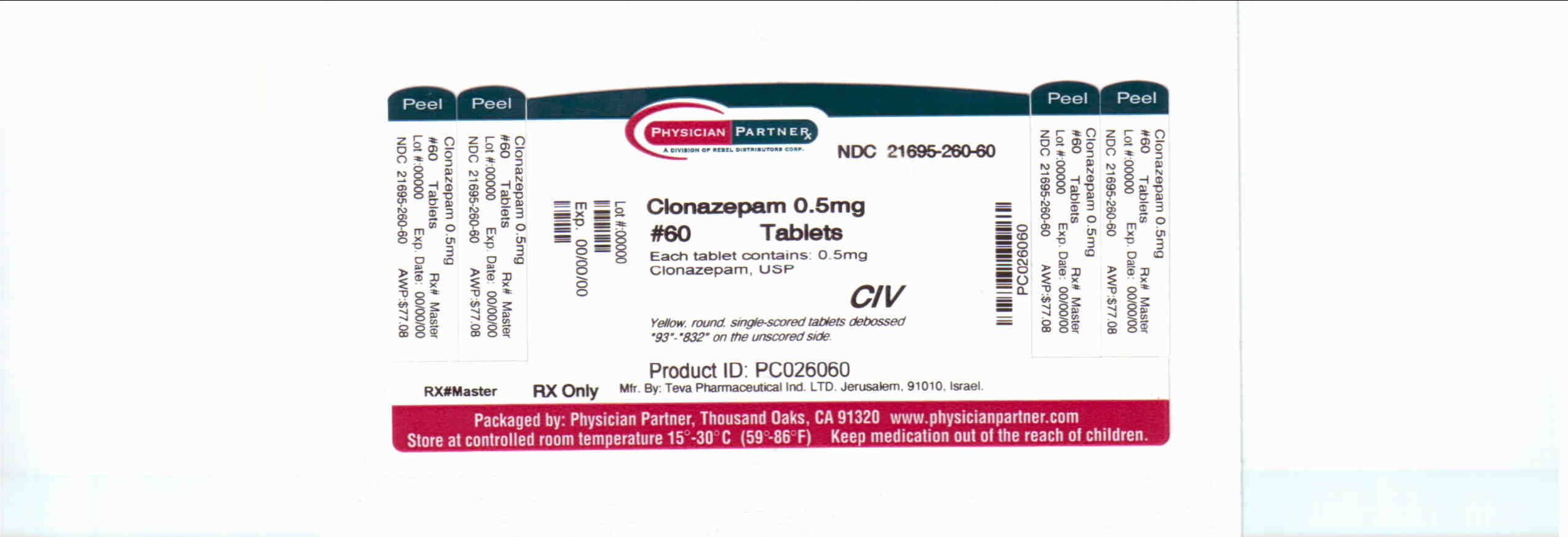 Source: www.recallguide.org
Source: www.recallguide.org
Clonazepam Information, Side Effects, Warnings and Recalls, On july 16th, 2024, endo usa, inc.
 Source: medium.com
Source: medium.com
Understanding Klonopin (Clonazepam) An InDepth Overview by paul ward Jan, 2024 Medium, The expanded recall involves clonazepam orally disintegrating tablets, with strength in usp 0.125 mg, 0.25 mg, 1 mg, and 2mg, packaged in cartons containing 60 tablets.
Category: 2024